Tritium: changing lead into gold
In order to produce energy from the fusion of light atoms, nature offers a dozen of possible combinations. Only one is accessible in the current state of technology: the fusion of hydrogen isotopes(1) deuterium and tritium.
But here's the challenge. While deuterium can be easily extracted from seawater, which contains 33 grams per cubic metre, tritium is much harder to source.
In nature, tritium is found only in trace amounts. The effect of cosmic rays on the outermost layers of the Earth's atmosphere produces anywhere from a couple of grams to a couple of kilograms every year (the estimates vary). A few dozen kilograms are also dissolved in oceans as a result of atmospheric nuclear testing carried out between 1945 and 1980.
Small quantities of tritium are also produced by CANDU-type nuclear reactors—on the order of 100 grams per year for a 600 MW reactor, or approximately 20 kilograms per year globally. This stock, unused today, will be enough to fuel ITER for the fifteen years of its deuterium-tritium campaign.
In the longer term, however, it will be necessary to develop solutions for the large-scale production of tritium. It is estimated that every fusion reactor will require on the order of 100 to 200 kilograms per year.
Nature, as if anticipating the problem, offers a solution that combines elegance and efficiency—the fusion reaction itself can produce the tritium that in turn will fuel the reaction. What's more, the process takes place within the vacuum vessel in a continuous, closed cycle.
When a deuterium nucleus fuses with a tritium nucleus inside of the fusion plasma, protons and neutrons are recombined into one helium atom and one neutron. The electric charge of the helium atom causes it to remain trapped within the magnetic cage that confines the plasma. The neutron, on the other hand, escapes at high speed and strikes the vacuum vessel walls, heating water that circulates under pressure and initiating a process that—in future reactors—will create electricity.
The neutron can serve another purpose, however. When it strikes an atom of lithium-6(2) it disrupts its building blocks (3 protons and 4 neutrons) and reorganizes them into one atom of helium (2 protons, 2 neutrons) and one atom of tritium (1 proton, 2 neutrons) while at the same time liberating energy.
From the point of view of the physics, then, the problem is solved—tritium can be produced within the tokamak if lithium is included in the walls of the vessel. It is now a matter of developing the technological solutions that will allow scientists and engineers to translate physics principles into a productive cycle of "tritium auto sufficiency" in tomorrow's fusion reactors.
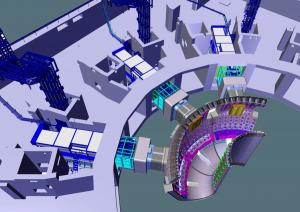
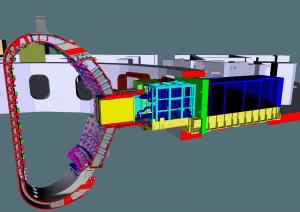
Luciano Giancarli has been interested in the question for nearly 30 years. At ITER, he heads the section that is in charge of the implementation of the Test Blanket Module (TBM) program—experimental blanket modules containing lithium that will be mounted inside the ITER vacuum vessel to test tritium breeding concepts. "The first challenge is the ratio between the neutrons generated by the fusion reaction and the tritium atoms actually produced," he explains. "For the system to work, the ratio must be higher than 1 meaning that—between the neutron and its lithium target—we'll need a 'neutron multiplier' like lead or beryllium.(3)"
The ITER Members have developed a number of concepts that will be tested in reactor-scale conditions in ITER. Although the TBM modules are all based on the same principle (the reaction between the neutron and lithium-6), each one is unique in its architecture, its structural materials, its cooling system, the form of its lithium (solid or liquid), and the manner in which the tritium will be extracted.
Inside of the ITER Tokamak, six spaces have been reserved for the breeding modules. Europe is planning two TBM systems; China, India, Japan and Korea are in charge of the four others. (As for the United States and Russia, they are participating in the program by supplying data that is important to the realization of the systems.)
Although the results of the tritium breeding experiments will be open to all Members, each provider will keep manufacturing details a secret due to the high commercial stakes linked to tritium production.
"We estimate that, in ITER operating conditions, the maximum productive capacity of each of the test modules will be on the order of 20 milligrams per day. In a commercial tokamak, this production will be on par with the power of the machine—on the order of 150 grams per day and per gigawatt," says Luciano.
The conceptual design phase for each of the TBM systems has now ended. Just like the other elements of the ITER machine, these tritium-breeding concepts will be dissected, analyzed and reviewed by a special committee before formal approval. Fabrication activities are planned to start in 2020.
To reach ITER goals and those, in a larger sense, for the future of fusion, the six TBM modules will play a fundamental role. By demonstrating their capacity to transform an element that is as common as lead on Earth (lithium) into the more rare and precious tritium, they will open the way to the industrial and commercial exploitation of fusion energy.
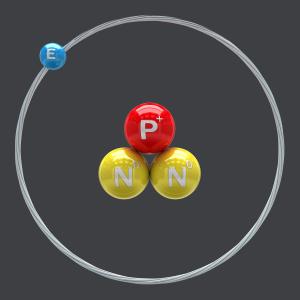
1 - Most elements of the periodic table exist in different forms called isotopes. The isotopes for a same element differ in the composition of their atomic nucleus. In a chemical reaction they act identically; in a nuclear reaction, isotopes can act in very different ways.
2 - Lithium-6 is a stable lithium isotope present in natural lithium at the level of 7.5%.
3 - When a neutron strikes an atom of lead or beryllium, its atomic structure is disturbed. After absorbing the neutron, the disrupted atom ejects two neutrons—this increases the number of neutrons available to generate, in a second step, tritium from the lithium-6 contained in the modules.